1.49 - 1.51 Covalent Giants
A valuable lattice
Activity. Diamond - close up
Enter your text here ...
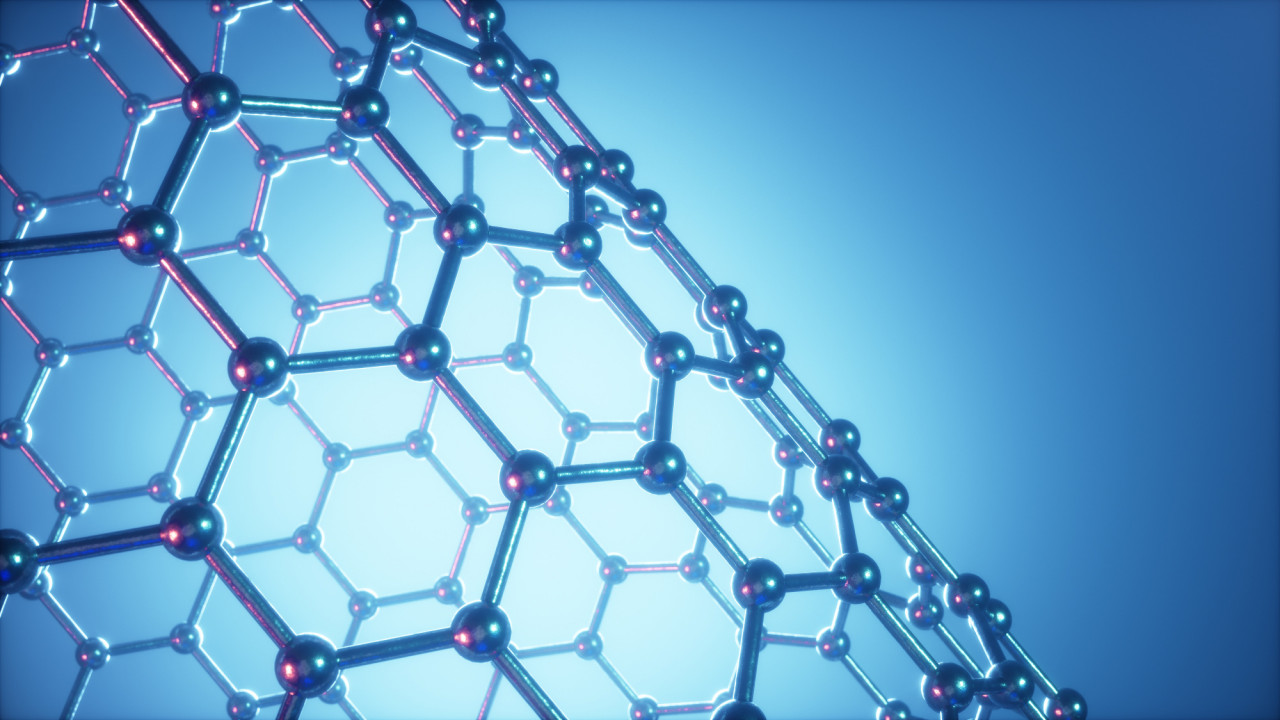
Click on the animation to explore this 3 dimensional visualisation of a giant covalent lattice by Surya.
The red spheres represent atoms of carbon. Each carbon atom has four identical covalent bonds. These each connect with four atoms which then connect with four more and so on - to form a giant covalent lattice.
Notice that in this structure when a carbon atom has 4 bonds all its outer electrons are involved in covalent bonds. There are no free electrons.
how to draw a diamond lattice
1.49 Giant covalent vs simple molecules
Students should:
- 1.49 explain why substances with giant covalent structures are solids with high melting and boiling points
- 1.50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness
- 1.51 know that covalent compounds do not usually conduct electricity
The structure formed is strong and requires a lot of energy to break it up and so the melting and boiling points of covalent lattices tend to be very high.
Enter your text here ...
Enter your text here ...
Enter your text here ...
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.