Extraction and uses of metals
Modern methods
Activity 1 : Phytomining and Bioleaching
Watch the video carefully, stopping where necessary to answer the questions below:
- Explain the main difference between high grade ore and low grade ore.
- What is happening to the supply of high grade copper ore?
- Explain what is meant by bioleaching
- What is the main advantage of using this method for metal extraction?
- What is phytomining?
- How do we release the metal which is extracted by phytomining
- What is the main disadvantage of using phytomining for metal extraction?
Enter your text here ...
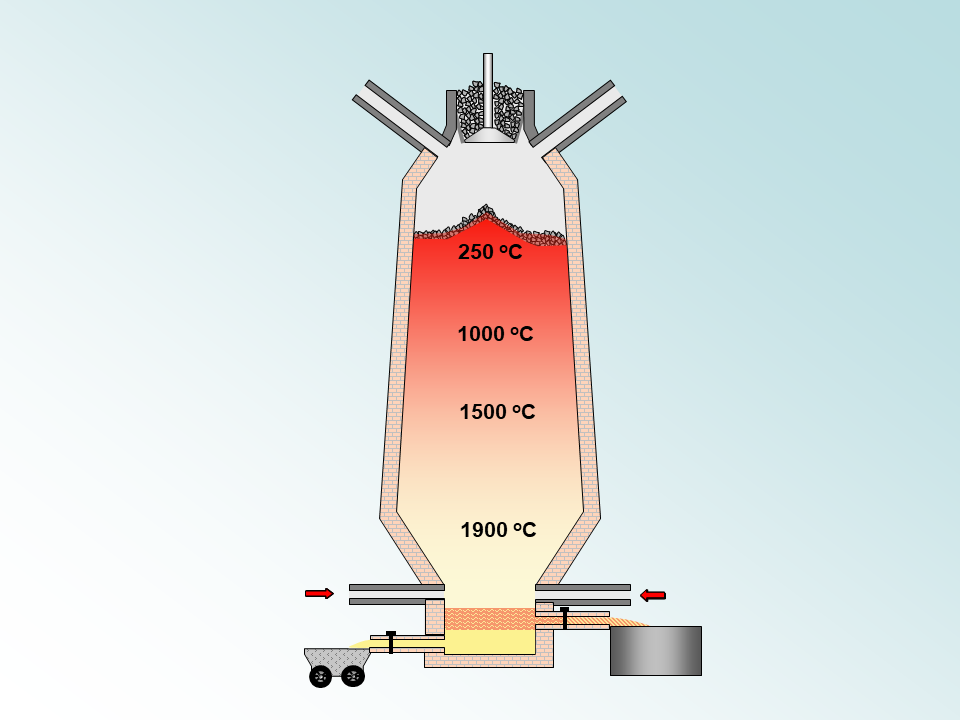
The Blast furnace
The blast furnace is the "original method" used for the extraction of iron from iron ore. It relies on the relative reactivity of carbon ( in coal) which reduces the iron oxide ( in iron ore) to metallic iron.
Limestone is added to react with the impurities in the ore . This decomposes and produces carbon dioxide gas.
The production of iron is not carbon neutral. Use chemical equations to explain why.
The thermit reaction is used to produce a plug of red hot iron which can be poured directly to join together two sections of rail on a railway track.
In the main reaction Iron III oxide and Aluminium react with one another. Pure iron is produced.
- Magnesium ribbon is used as the fuse.
- Write an equation for the reaction of magnesium with the oxygen in the air
- Try to write a word equation for this reaction. What is reduced in this reaction?
- What is oxidised?
Enter your text here ...