1.31 Finding formulae
Finding the formula of a compound is important to chemists. We can often predict what the formula might be because we know something about the electron configurations and the way atoms reorganise their electrons to achieve full or empty outer shells.
However, here we look at a number of reactions where accurate mass measurements enable us to prove experimentally, the formula of a compound.
Students should:
1.31 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water and salts containing water of crystallisation
1.31 Activity 1. Finding formulae
Make a copy of the table below and use it to record the mass measurements shown in the video.
| reading | items measured | mass in g |
| 1 | crucible | |
| 2 | crucible + magnesium | |
| 3 | crucible + magnesium oxide | |
| 4* | magnesium | |
| 5* | magnesium oxide | |
| 6* | oxygen | |
* calculations: to find the mass of:
- magnesium used: ( reading 2 - reading 1)
- magnesium oxide formed: (reading 3 - reading 1)
- oxygen combined ( reading 5 - reading 4 )
Enter your text here ...
| element | Magnesium | Oxygen |
| Mass /g | 0.570 | 0.336 |
| Ar | 24.3 | 16.0 |
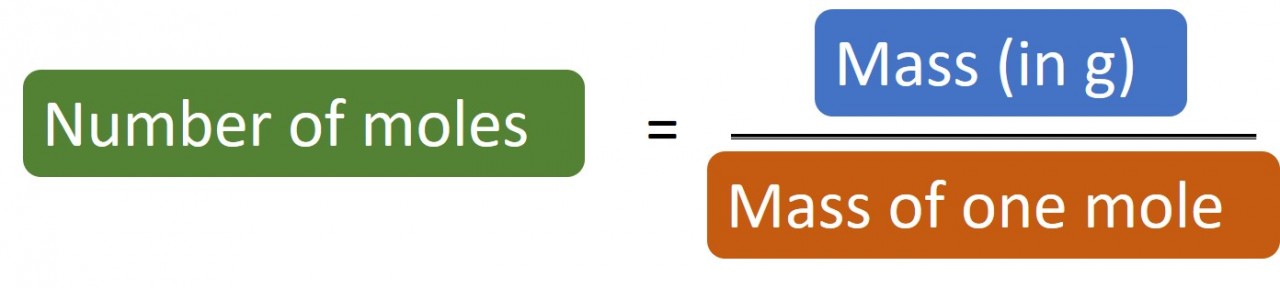
| moles ( mass/Ar) | 0.0235 | 0.0210 |
| mole ratio (approx) | 1 | 1 |
Once you've calculated the number of moles - look for the simplest ratio . In this case it is 1:1.
So the formula can now be written as MgO
Enter your text here ...
Use the animation here to calculate the formula of copper oxide.