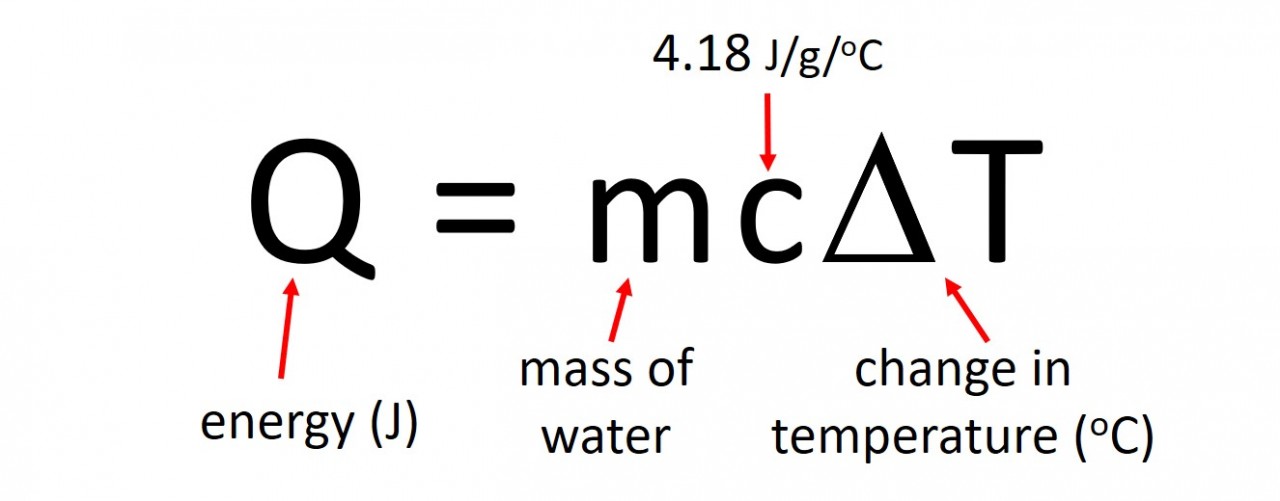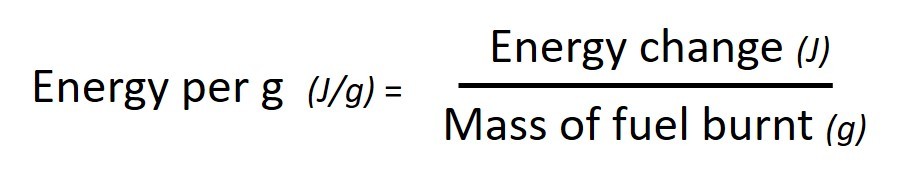3.1 - 3.8 Energetics
3.1 Energetics
Students should:
- 3.1 know that chemical reactions in which heat energy is given out are described as exothermic, and those in which heat energy is taken in are described as endothermic
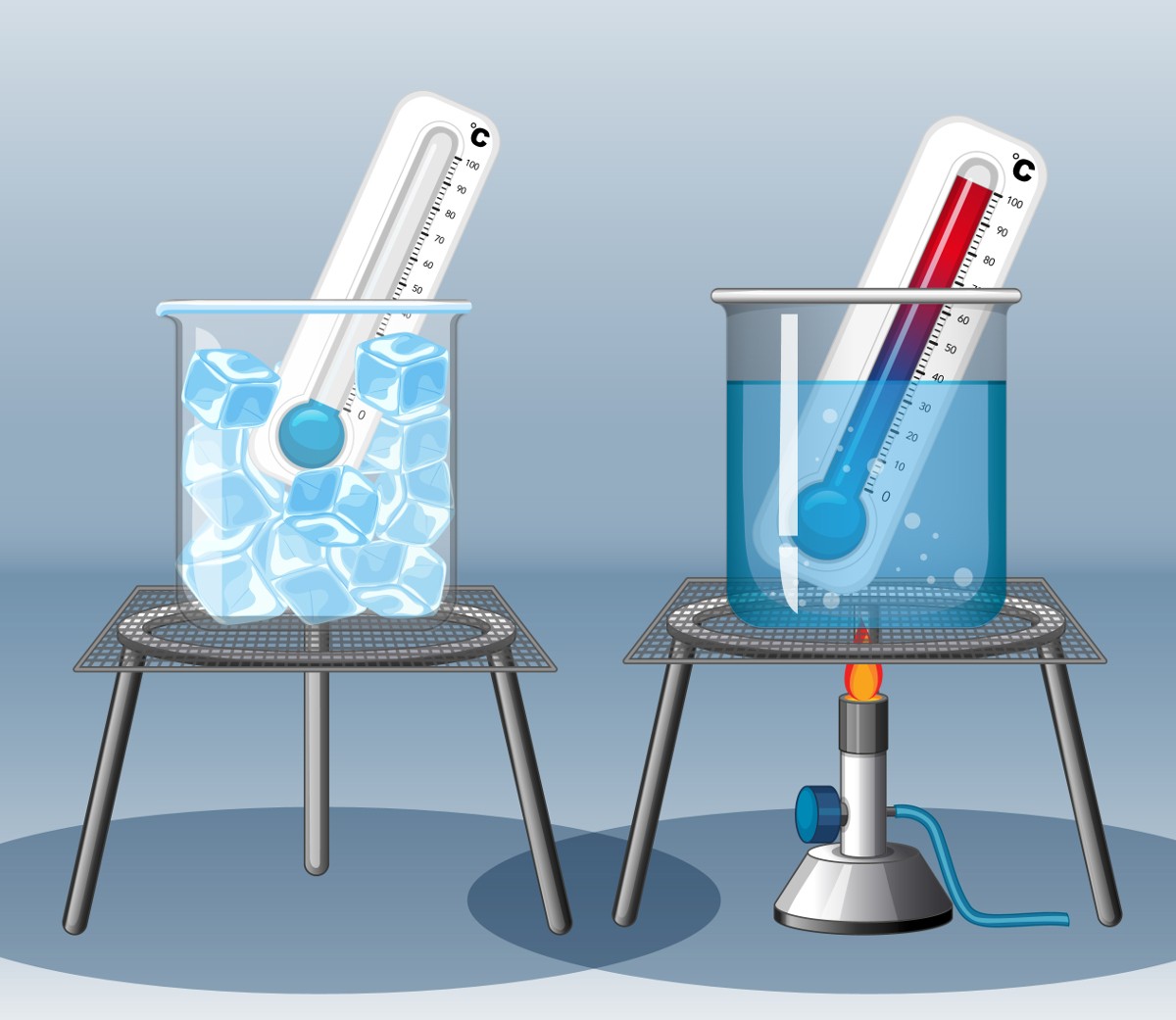
Every chemical reaction has an accompanying change in energy.
Some reactions release energy to the surroundings and are known as Exothermic.
Reactions which take in energy from their surroundings are known as Endothermic
When a chemical reaction releases energy in a useful form the substance involved can be regarded as a fuel
3.2 Activity 2. Coke can calorimetry
This experiment uses a diet coke can as a calorimeter.
Students should:
- 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation
Watch this very brief video and note down the key measurements taken:
| 1. mass of water heated | |
| 2. initial mass of nut | |
| 3. final mass of nut ( after burning) | |
| 4. mass change (3-2) | |
| 5. initial temperature of water / 'C | |
| 6. final temperature of water / 'C | |
| 7. temperature change (6-5) |
| 1. mass of water heated | 100ml |
| 2. initial mass of nut | 6.75g |
| 3. final mass of nut ( after burning) | 5.79g |
| 4. mass change (3-2) | 0.96g |
| 5. initial temperature of water / 'C | 22.0oC |
| 6. final temperature of water / 'C | 50.0oC |
| 7. temperature change (6-5) | 28.0oC |
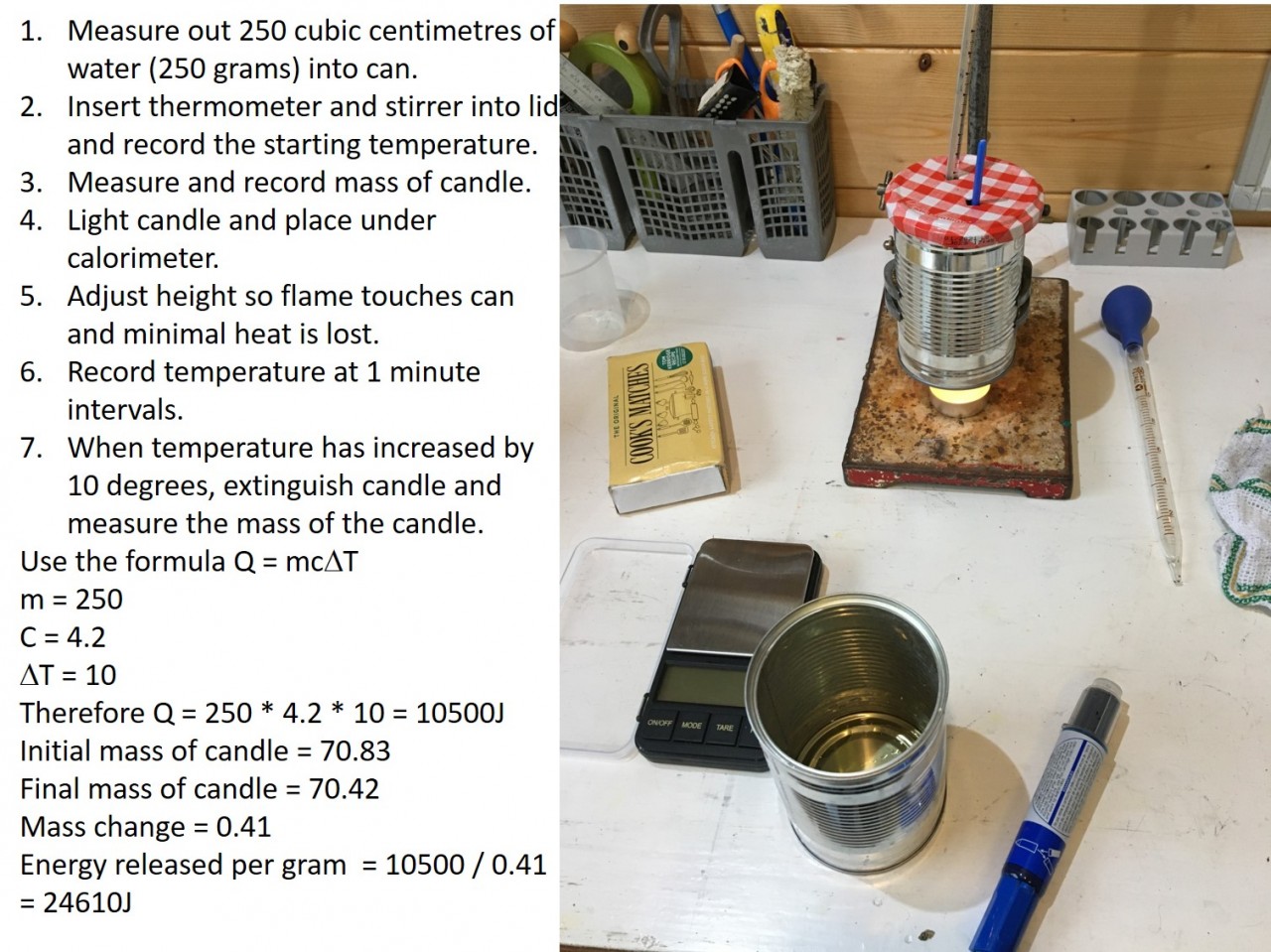
3.2 Activity 3 - Candle power
3.3 Activity 3. Do the maths
3.4 Activity 4. How much per mole?
Students should:
- 3.4 calculate the molar enthalpy change ("delta"H) from the heat energy change, Q
The energy produced by a particular reaction can be found out using calorimetry . If we measure the mass of fuel before and after the burning we can find out the energy released per gram by using the following calculation:
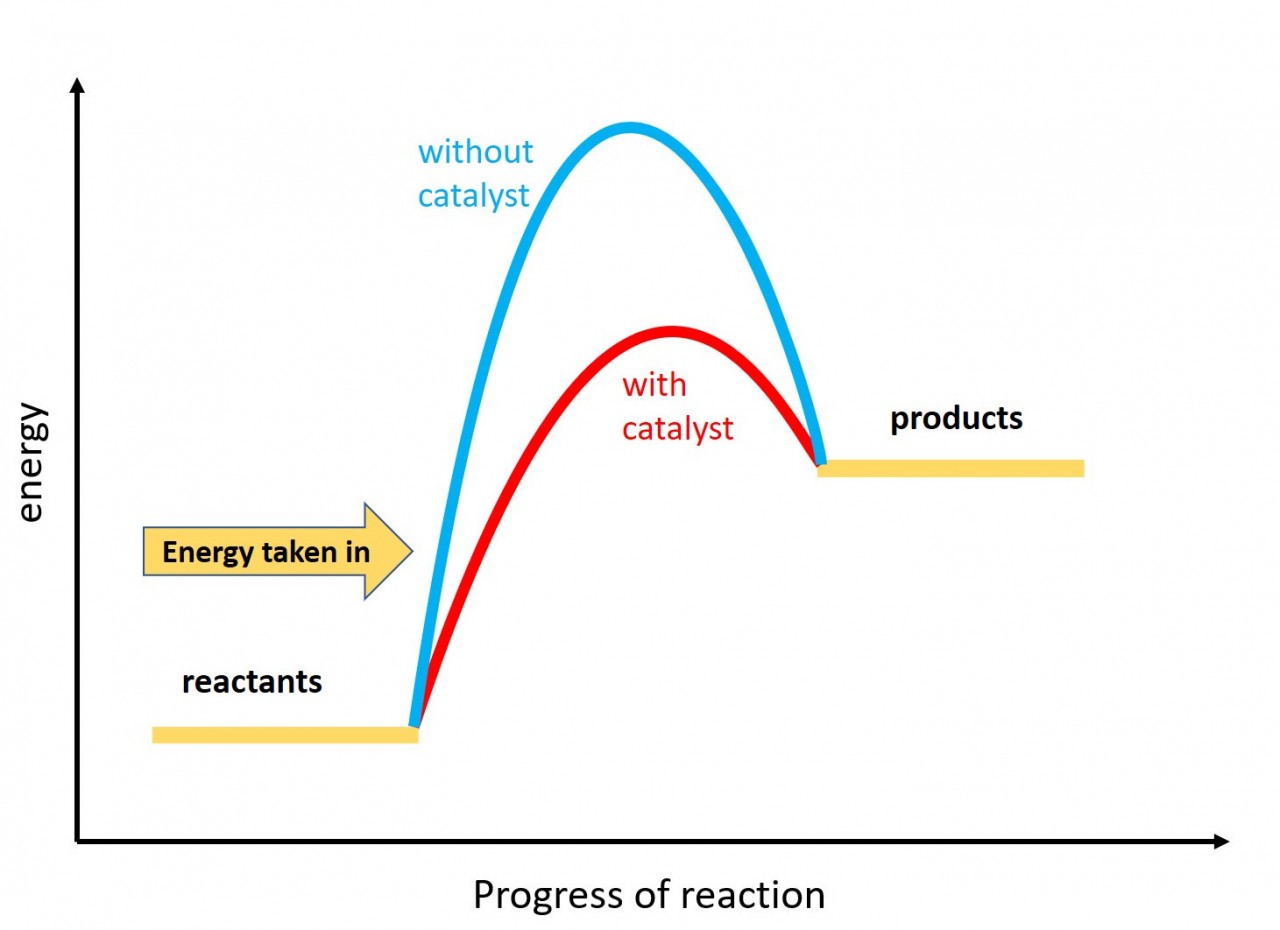
3.5 Activity 1. Exo and Endo
Students should:
- 3.5 draw and explain energy level diagrams to represent exothermic and endothermic reactions
When a reaction takes in energy from the surroundings it is known as an endothermic reaction.
Use this idea and the exothermic reaction energy profile to draw an energy profile for an endothermic reaction.
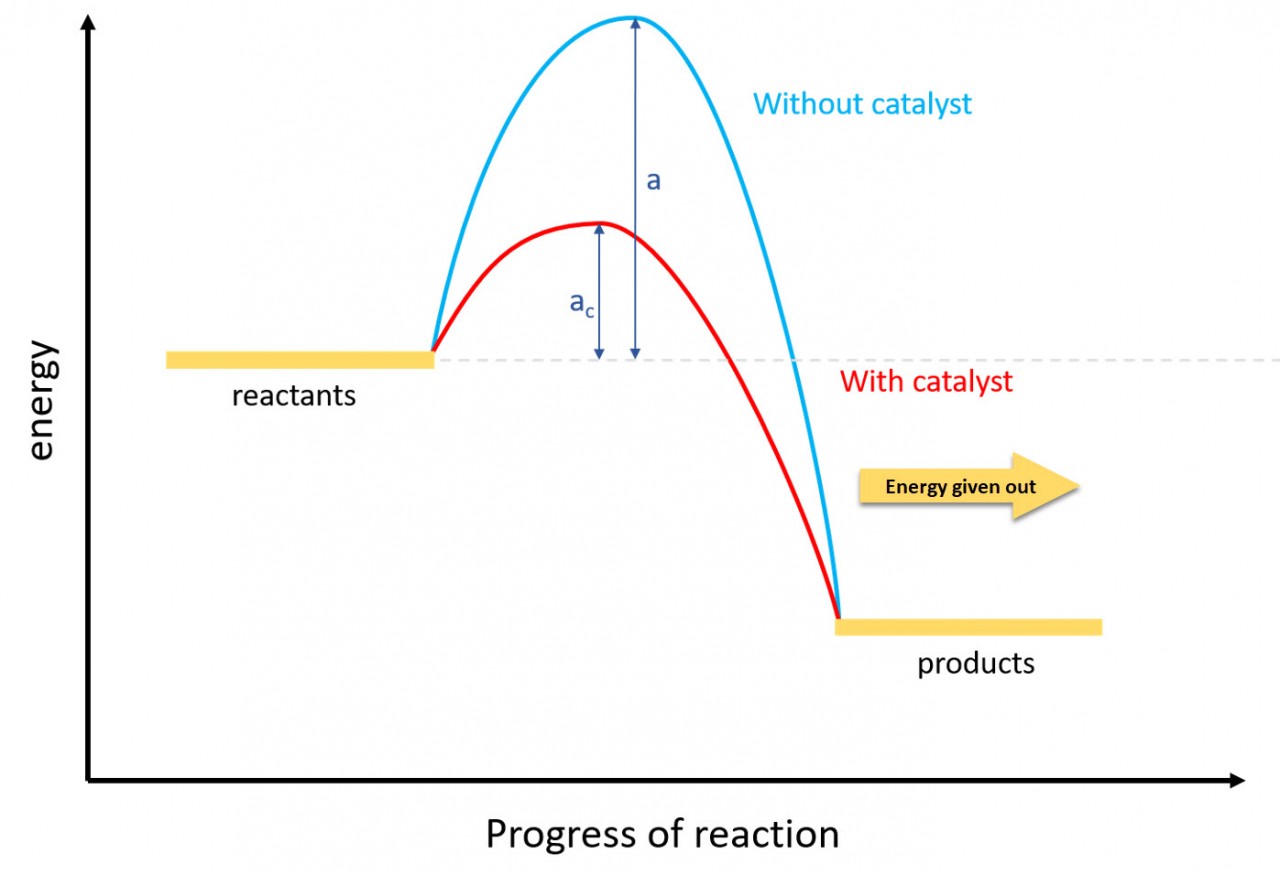
3.6 Activity 5. Profit and loss
Students should:
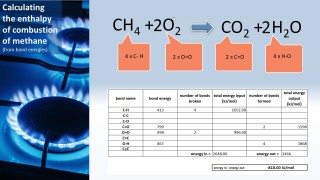
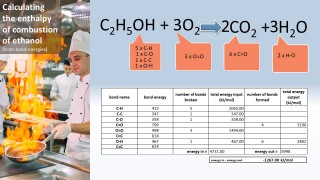
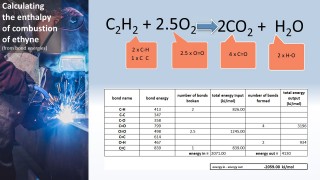
- 3.6C know that bond-breaking is an endothermic process and that bond-making is an exothermic process
- 3.7C use bond energies to calculate the enthalpy change during a chemical reaction
Use the animation to see how the formation of a covalent bond releases energy and is therefore an exothermic process. Notice that the energy released in this case is 432 kJ per mol.
That same amount of energy (if supplied) would be sufficient to break the bond.
We can use the fact that individual bonds have specific amounts of energy associated with them to calculate an approximate value for the energy change for a chemical reaction: