1.29 Calculating masses
Magnesium is a highly reactive metal which reacts violently with oxygen to produce a white solid called magnesium oxide.
This can be shown as follows:
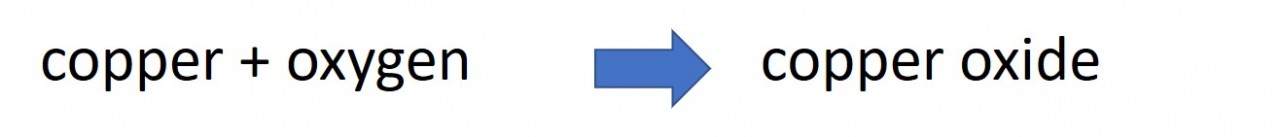
Chemical equations are a useful shorthand way of describing the changes that take place when a chemical reaction happens. Word equations can be helpful for example :
It is often more helpful to use the chemical formulae of the reactants since the formulae show the actual elements involved:
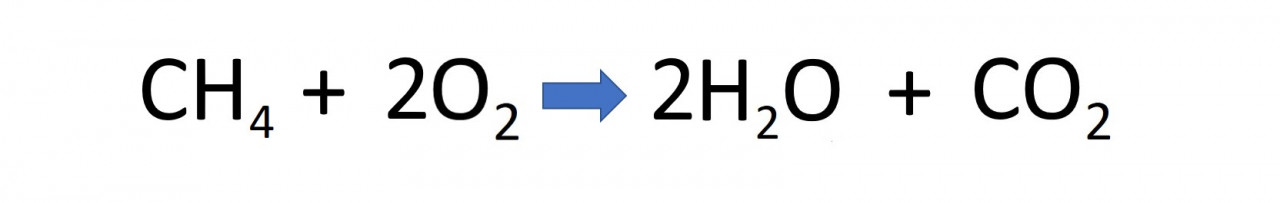
For example if you were predicting a word equation for when methane burns in oxygen you might suggest:
This is wrong of course because methane is not a single element, it is a compound of carbon and hydrogen (CH4). When it burns in a plentiful supply of oxygen (O2) the carbon in methane becomes carbon dioxide (CO2) and the hydrogen becomes water (H2O). This is more easily shown as a symbol equation:
1.29 Conserving mass
Students should:
1.29 calculate reacting masses using experimental data and chemical equations
Use the animation to see how to balance the equation for the combustion of methane.
Notice that when it is balanced, the equation shows that the total mass of the reactants is equal to the total mass of the products.
The law of conservation of mass states that the mass of the products in a chemical reaction must equal the mass of the reactants
We use this idea throughout the course.
1.29 Activity 1. Masses reacting
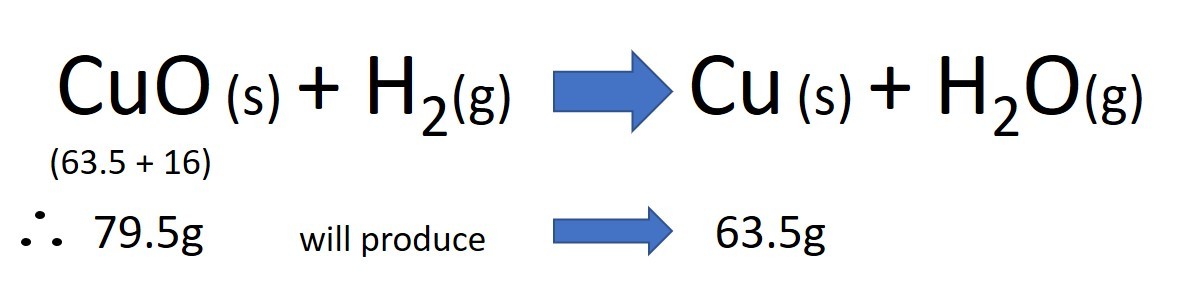
The video here shows a classic reaction where hydrogen gas (H2) is used to remove oxygen from copper (II) oxide (CuO). The reactants are therefore hydrogen and copper oxide. The products are water and copper.
Study the video closely. You will see that the black copper oxide slowly becomes copper coloured as the oxygen is removed from it. The water produced in this reaction leaves the tube as vapour and so the mass of the tube decreases as the reaction continues:
1.29 Keep it in proportion
The combustion of methane in oxygen produces carbon dioxide gas as well as water vapour. This exercise allows us to calculate the "carbon footprint" produced by the burning of this fossil fuel.
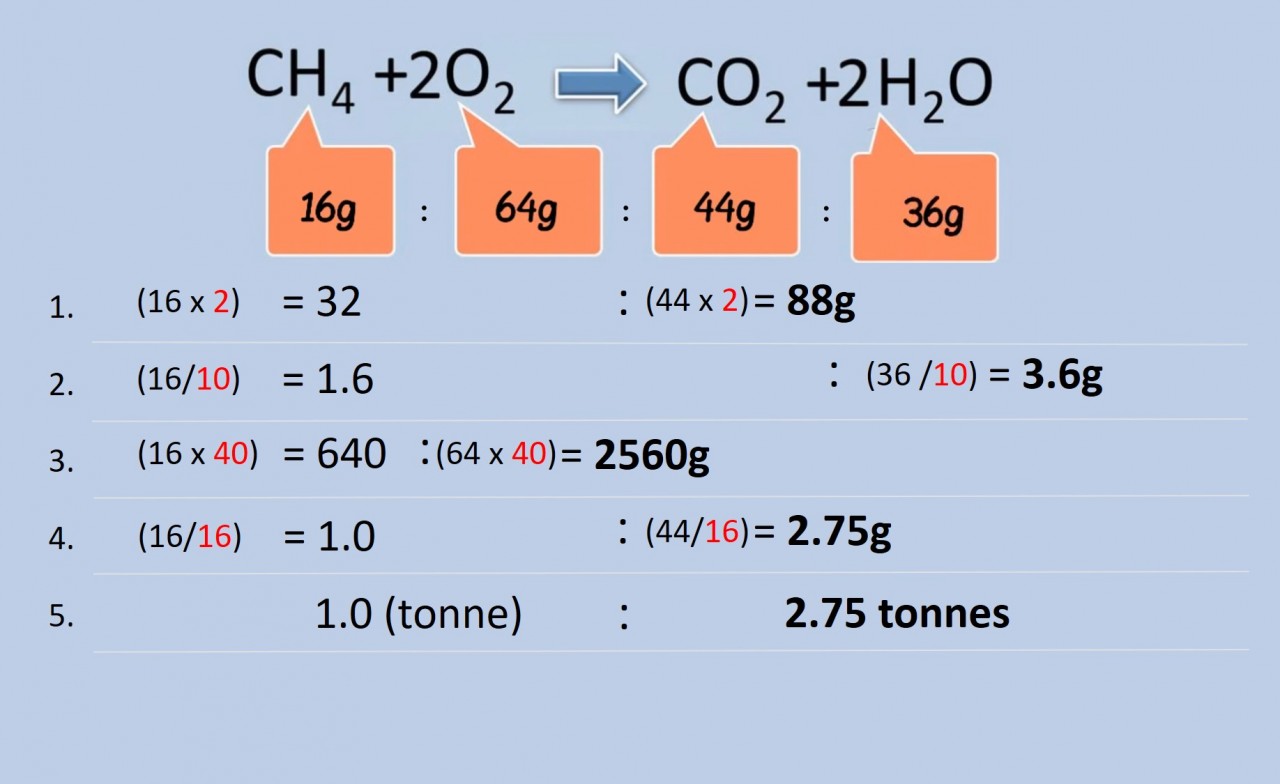
Use the equation and the relative mass data in the video to calculate the following:
the mass of:
- carbon dioxide produced when 32g methane is burnt in excess oxygen?
- water produced when 1.6 g methane is burnt in excess oxygen?
- oxygen required for the complete combustion of 640g methane?
- carbon dioxide produced by the complete combustion of 1g of methane
- carbon dioxide produced by the complete combustion of 1 tonne of methane?