2.28 Acids, alkalis and titrations
Acids and alkalis are all around us; many everyday foods, drinks, cleaning products are acidic or alkaline.
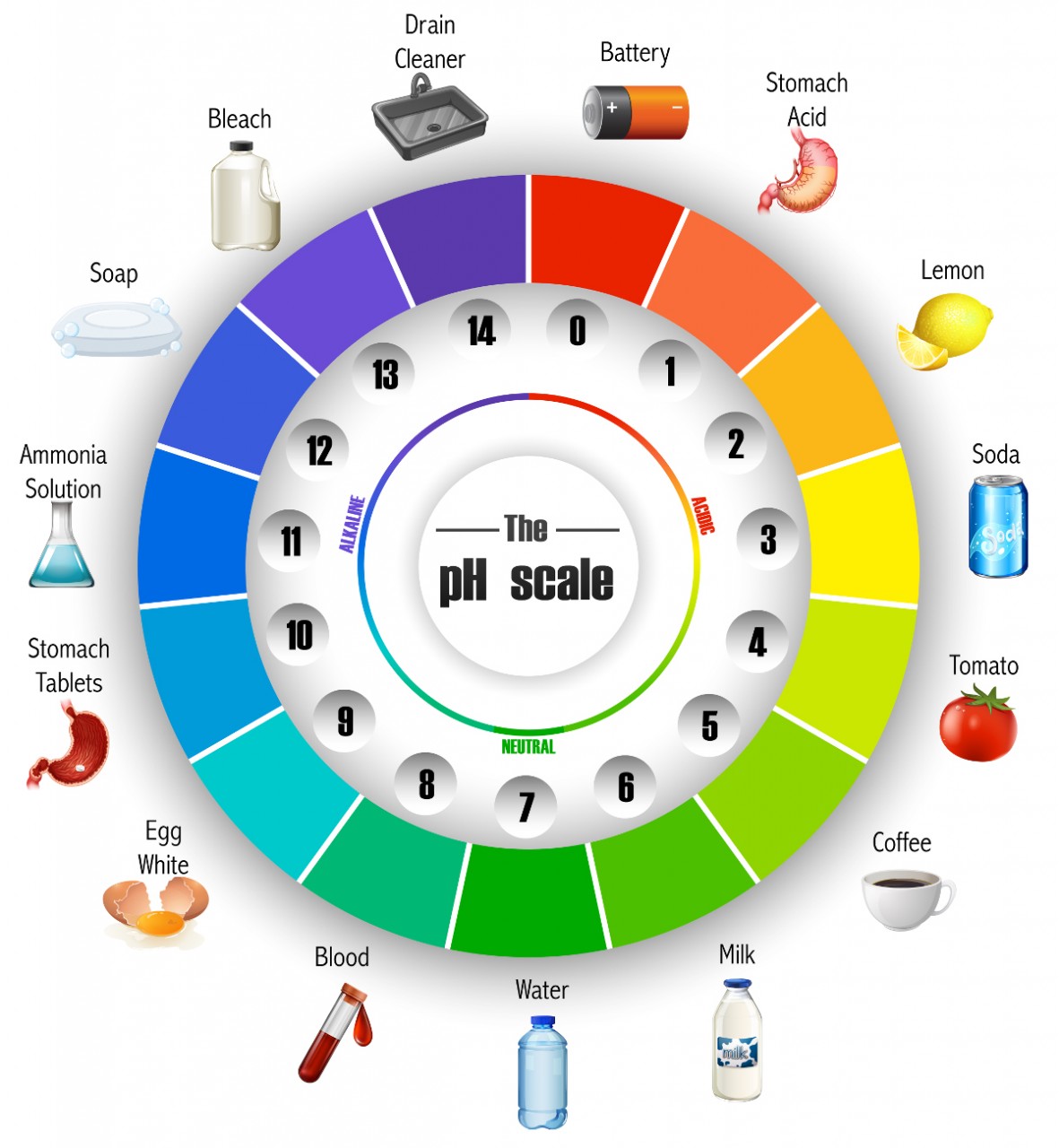
Study the image of the pH scale "wheel". This scale gives a measure of the acidity or alkalinity of a substance. A value of pH 7 is regarded as neutral . Substances with a pH value greater than 7 are regarded as alkaline and those with pH value of less than 7 are acidic.
Acids can be neutralised by alkalis and alkalis can be neutralised by acids.
use the information on the pH colour wheel and your general knowledge to figure out some general properties of acids and compare them with alkalis:
Think about :
- taste of the substance
- effect it might have on your skin
- whether or not the substance is safe to drink or eat
- how the substance behaves in water
All acids taste sour and have a pH of <7. Acids can neutralise bases if they have an equal amount of moles. they are corrosive and are soluble in water.
Examples of acids are:
- Battery pH 0
- Stomach Acid pH 1
- Lemon pH 2
- Soda pH 3
- Tomato pH 4
- Coffee pH 5
- Milk pH 6
All alkalies feel "soapy and have a pH > 7. they neutralise acids and are corrosive.
Examples of alkalies are:
- Drain cleaner pH 14
- Bleach pH 13
- Soap pH 12
- Ammonia solution pH 11
- Stomach tablets pH 10
- Egg whites pH 9
- Blood pH 8
2.28 Activity 1. The Acid test
Use this simulation to find out the approximate pH of the range of substances available in the drop down list.
Prepare a table of results to record the pH values
2.28 -2.30 Activity 2. Using indicators
Students should:
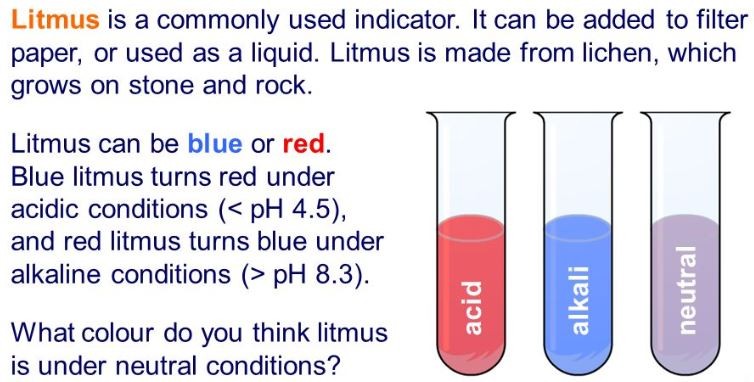
- 2.28 describe the use of litmus, phenolphthalein and methyl orange to distinguish between acidic and alkaline solutions
- 2.29 understand how to use the pH scale, from 0–14, can be used to classify solutions as strongly acidic (0–3), weakly acidic (4–6), neutral (7), weakly alkaline (8–10) and strongly alkaline (11–14)
- 2.30 describe the use of universal indicator to measure the approximate pH value of an aqueous solution
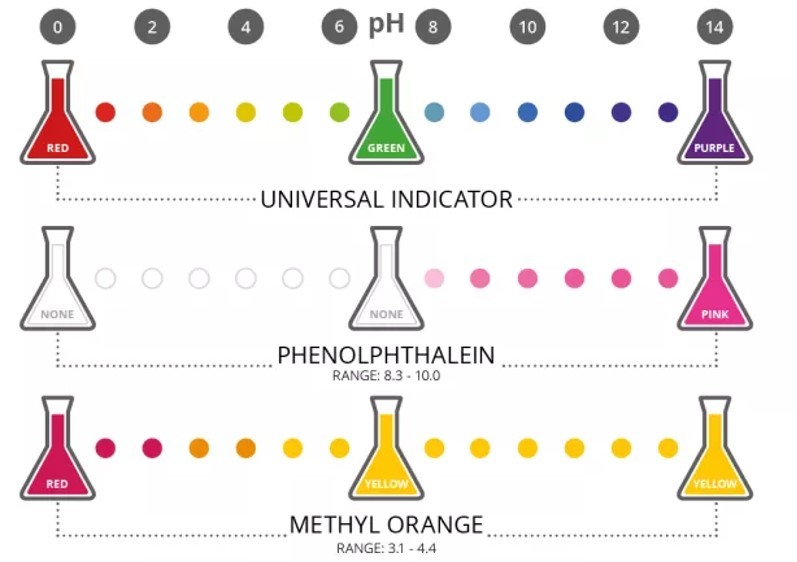
Use the images to copy and complete the table below. Once completed, you should learn the colour changes for each indicator in each condition.
| INDICATOR NAME | colour in acid (pH1) | colour in water (pH7) | colour in alkali (pH14) |
| Universal indicator | |||
| Phenolphthalein | |||
| Methyl Orange | |||
| Litmus |
| INDICATOR NAME | colour in acid (pH1) | colour in water (pH7) | colour in alkali (pH14) |
| Universal indicator | Red | Green | Purple |
| Phenolphthalein | Colourless | Colourless | Pink |
| Methyl Orange | Red | Yellow | Yellow |
| Litmus | Red | Purple | Blue |
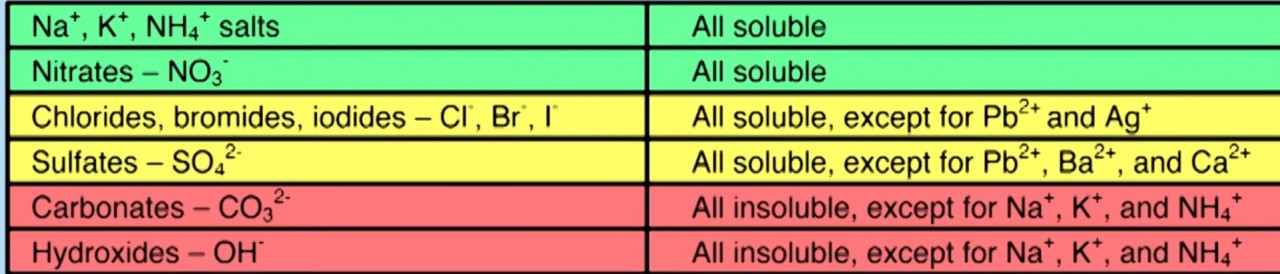
Solubility rules
2.28 - 2.30 Activity 3. Remember the colours. Complete the matching exercise below
2.31 Activity 4. Neutralisation
Students should:
- 2.31 know that acids in aqueous solution are a source of hydrogen ions and alkalis in a aqueous solution are a source of hydroxide ions
- 2.32 know that alkalis can neutralise acids
Work your way through the animation .
Note down the formulas and names of the acids involved.
- List the names and formulae of the acids listed.
- What is the name of the ion found in all acids.
- What is the ion found in the alkaline solution?
- Sulfuric acid = H2SO4, Hydrochloric acid = HCl, Nitric acid = HNO3.
- Hydrogen ion H+(aq)
- Hydroxide ion OH-(aq)
2.33 Activity 5. Doing titrations
Students should:
- 2.33C describe how to carry out an acid-alkali titration
Titration is a laboratory technique which is used to to measure accurately the volume of a solution required to react completely with another solution.
If one of the solutions is of unknown concentration, the titration results can be used to do calculations to find it out.
The solution( of unknown concentration) is measured out accurately into a conical flask and a suitable indicator is added. The solution of known concentration is then added in measured amounts until the indicator changes.
Watch both videos and answer the questions below:
- Name the apparatus which is used to measure out accurately a volume of unknown solution?
- What apparatus is used to add the solution of known concentration to the flask?
- Why is an indicator solution added to the contents of the flask?
- Why do you think it is necessary to repeat the process several times?
- Volumetric pipette
- Burette
- A change in colour provided by the indicator solution indicates when the solution is neutralised
- To ensure a fair result and remove any anomalous data