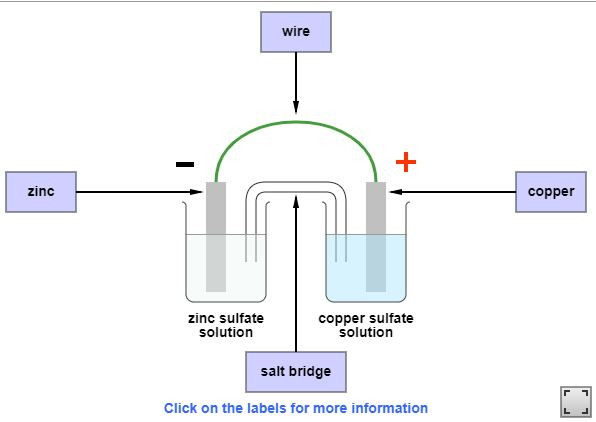
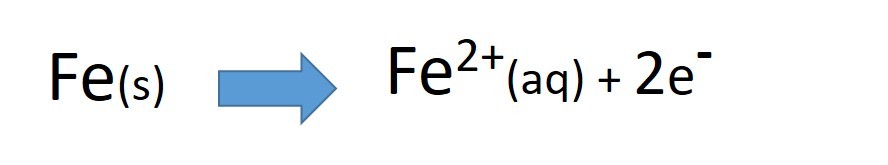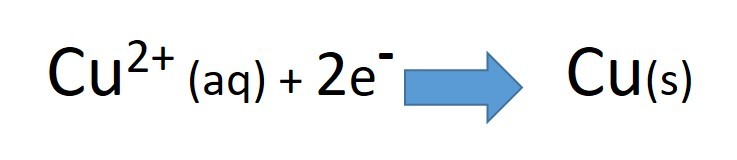The Reactivity Series
2.15 - 2.20 Loss and gain
Oxidation and reduction reactions are very commonplace. When a element combines with oxygen that element is said to have been oxidised. When the oxygen is removed from an oxide of an element to leave the element on its own, the oxide is said to have been reduced.
Here we will use broader definition of oxidation and reduction which considers the loss and gain of electrons.
Oxidation Is the Loss (of electrons) Reduction Is the Gain (of electrons)
2.15 Activity 1. Displacement
Students should:
Enter your text here ...
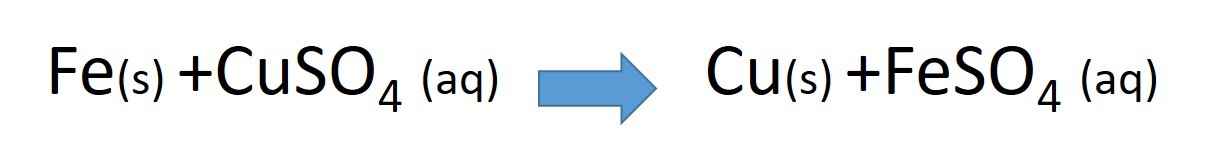
This video explains the reaction when copper metal is displaced from solution by the more reactive metal iron. An iron nail is placed in a solution of copper sulphate.
- which is the more reactive metal
- what metal is displaced from solution?
- which ions are reduced
- explain what is being oxidised in this reaction?
A question of reactivity
Video Exercise: Metal displacement reactions close up..
This video shows a number of metal displacement reactions magnified and speeded up. For each reaction in turn:
- decide which metal is oxidised
- decide which metal is reduced
- write two half equations to represent these changes
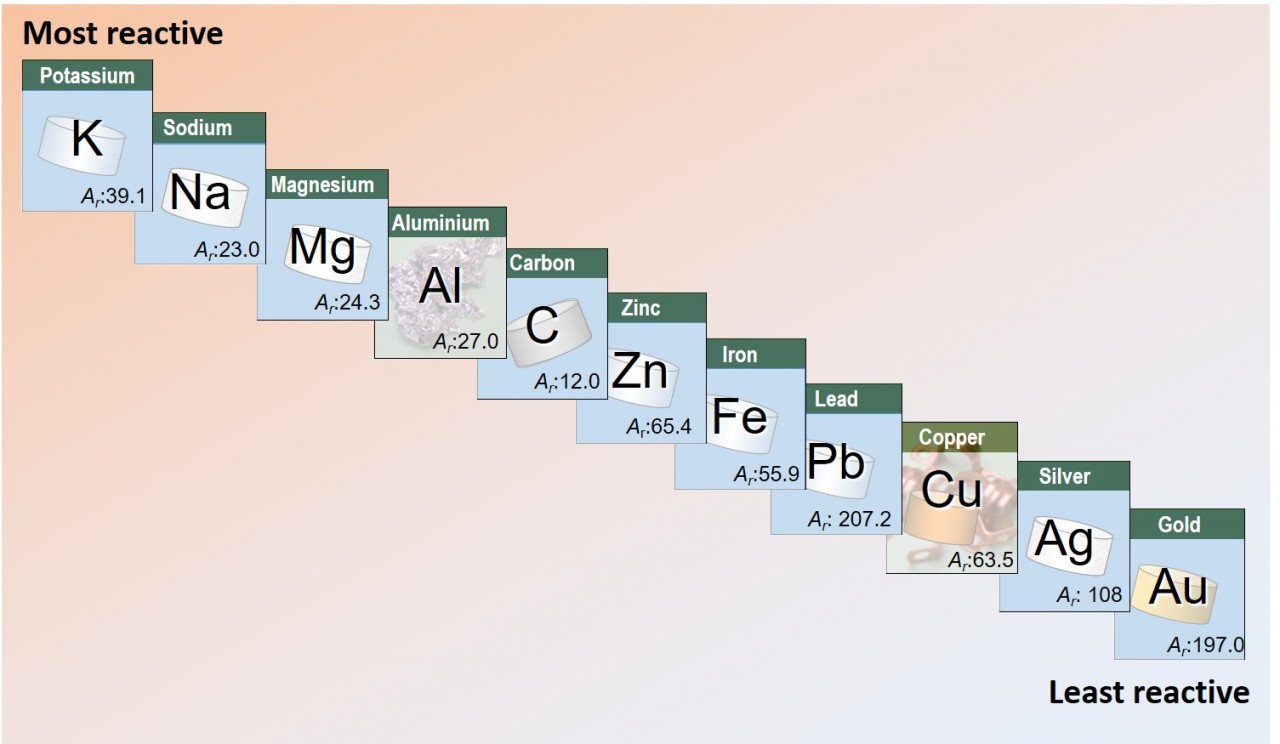
- use these equations to decide the order of reactivity of lead, silver, copper and zinc