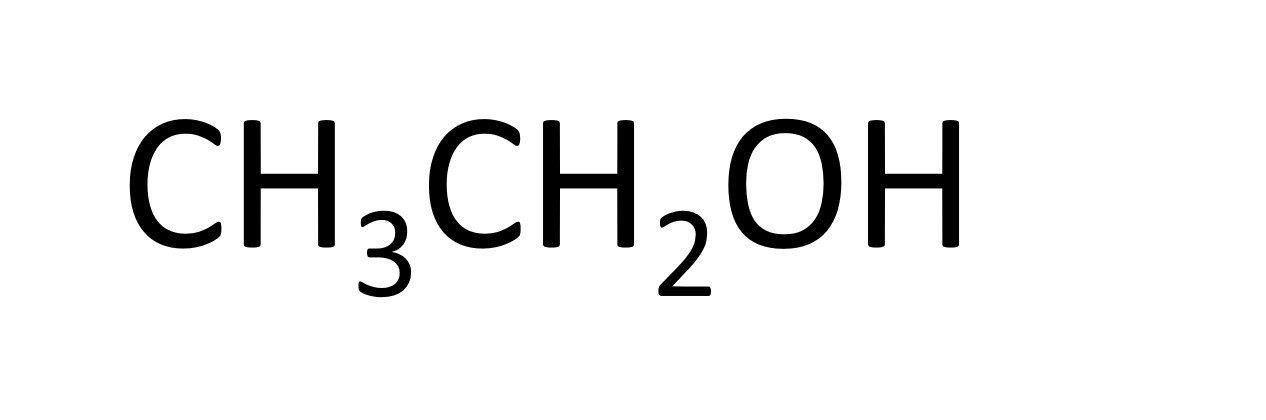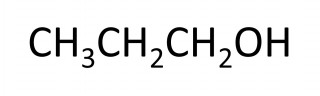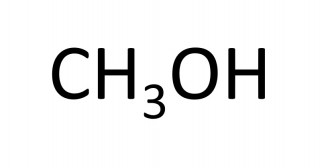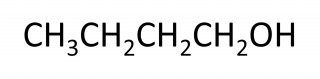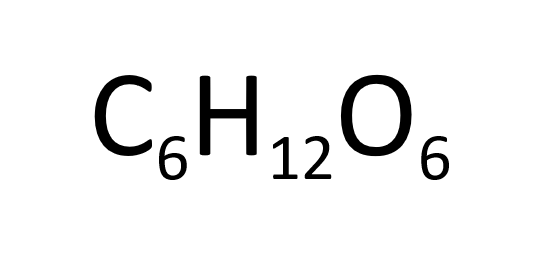4.29 - 4.33 Alcohols
4.29. Handle with care
The alcohols are another homologous series like the alkanes and the alkenes. Alcohols contain a functional group made up of an oxygen atom and a hydrogen atom.
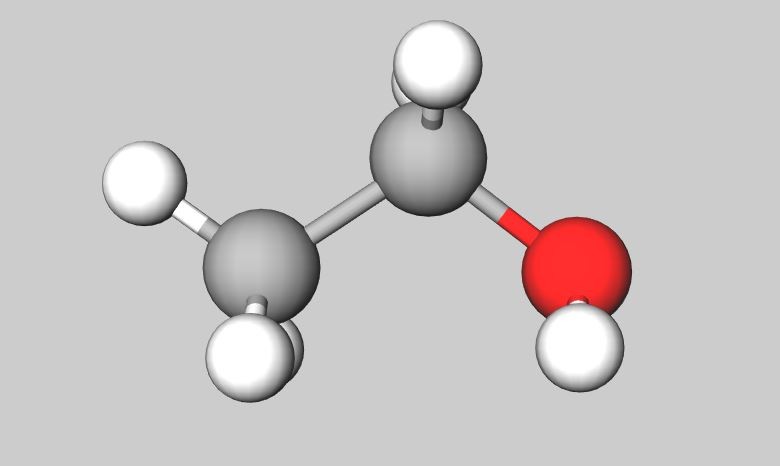
Ethanol is one member of the alcohol series. This is the alcohol in alcoholic drinks. Alcohols are good at dissolving things and are therefore sometimes used as solvents. Alcohols will also burn well and can therefore be used as fuels.
Assumed background knowledge:

4.1 - 4.7 Organic chemistry
4.29 - 4.30 Activity 1. Meeting "OL"
Students should:
- 4.29C know that alcohols contain the functional group −OH
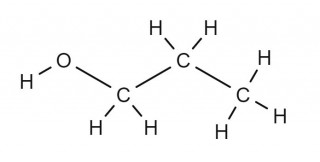
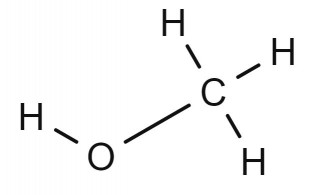
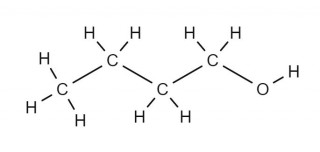
- 4.30C understand how to draw structural and displayed formulae and name methanol, ethanol, propanol and butanol
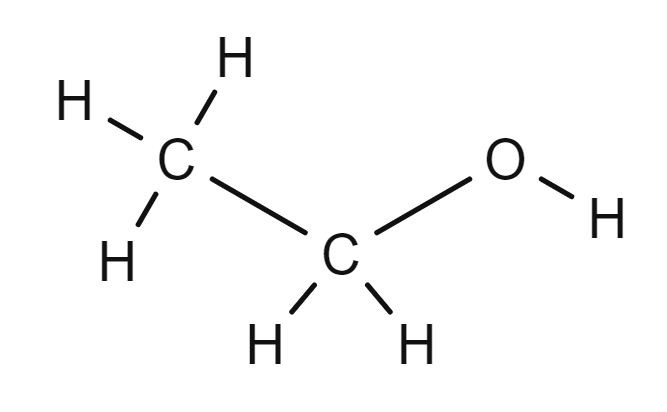
The functional group in an alcohol is the -OH group. This group gives the suffix "ol" to the name of the alcohol. The prefix used is the same as usd for the alkanes ; methan.., ethan.. , propan..., butan.. etc.
The names of the first four members of the homologous series of the alcohols are methanol, ethanol, propanol, butanol.
Can you draw the structural formula and the displayed formula for each alcohol? Check your answers against the diagrams on the other two tabs.
The names of the first four members of the homologous series of the alcohols are methanol, ethanol, propanol, butanol.
4.31 Activity 2. Oxidising ethanol - three ways.
Students should:
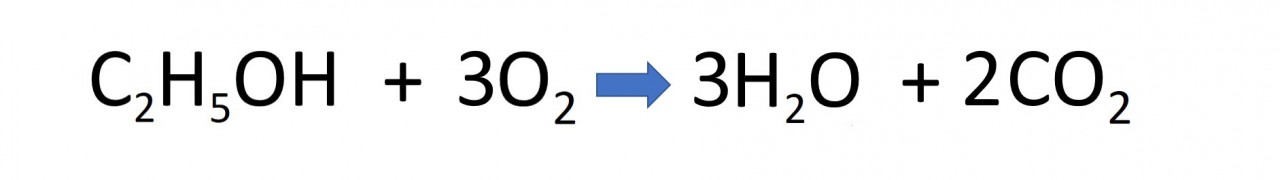
- 4.31C know that ethanol can be oxidised by: burning in air or oxygen (complete combustion)
Ethanol burns easily in the air. It burns cleanly and releases energy in a useful form, Thus ethanol can be used as a fuel.
Ethanol is quite a clean fuel and if made by the fermentation of sugar, it can be regarded as a renewable. However, it is expensive and its production can use up land and resources which otherwise could be used for food production.
Students should:
- 4.31C know that ethanol can be oxidised by: reaction with oxygen in the air to form ethanoic acid (microbial oxidation)
When alcohols are oxidised they can produce a type of acid known as a carboxylic acid. One example of a carboxylic acid is ethanoic acid . This is also known as Acetic acid and is the acid found in vinegar.
Acetic acid bacteria are found in grapes. When oxygen is present these bacteria will cause the oxidation of alcohol ( ethanol) to acetic (ethanoic) acid. Since bacteria cause this change to happen, this process can be described as microbial oxidation.
This explains why if a bottle of wine is left open to the air for too long, the alcohol ( ethanol) in it gets oxidised ( to ethanoic acid) and can become acidic and tastes unpleasant.
Students should:
- 4.31C know that ethanol can be oxidised by: heating with potassium dichromate(VI) in dilute sulfuric acid to form ethanoic acid
When alcohols are oxidised they can produce a type of acid known as a carboxylic acid. One example of a carboxylic acid is ethanoic acid . This is also known as Acetic acid and is the acid found in vinegar.
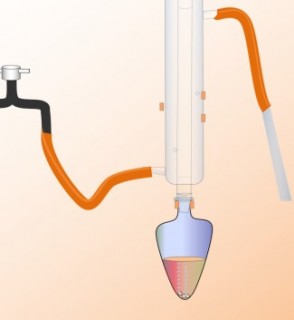
We can make carboxylic acids in the laboratory by mixing an alcohol with an oxidising agent and heating it up. The first four minutes of this video show the apparatus used.
Notice that the condenser is mounted vertically on top of the flask.
Notice the colour changes which take place as the reaction proceeds.
4.32C Activity 3. Making ethanol - two ways
Students should:
- 4.32C know that ethanol can be manufactured by one of two methods involving:
Watch the video and read the information in each of the tabs and use it to complete the summary table:
This method of ethanol production uses fossil fuels and needs a high temperature and pressure.
The reaction is fast and the ethanol produced has a high purity.
Glucose is fermented by yeast in anaerobic conditions to produce ethanol and carbon dioxide.
This reaction happens at low temperatures and uses renewable resources. However the ethanol produced needs to be purified by distillation.
| Addition of steam to ethene | Fermentation | |
cost of raw materials |
||
| renewability of raw materials | ||
| energy requirements | ||
| speed of reaction | ||
| purity of ethanol produced |
4.33C Activity 4. Optimium conditions
Students should:
- 4.33C understand the reasons for fermentation, in the absence of air, and at an optimum temperature
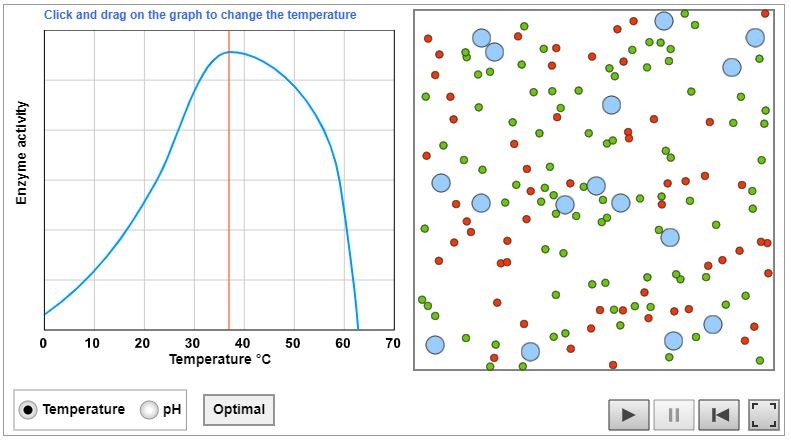
The fermentation of glucose is catalysed by an enzyme in yeast. Enzymes are delicate molecules which will change shape if their temperature gets too high. When this happens the enzyme becomes ineffective. The enzyme is said to have become denatured. The graph shows the temperature at which this happens.
If the fermentation of glucose takes place in the presence of air the products are carbon dioxide and water.
Click on the graph to see the animation. Use the animation to find the optimum temperature for this enzyme.
Enter your text here ...