1.14 Atoms and molecules
Atomic models
Atoms are the building blocks of matter. Atoms are the smallest possible particles into which an element can be subdivided, without changing its properties.
As building blocks, they can be thought of spheres.
However, to understand how and why atoms join up with one another, a more sophisticated model is needed..
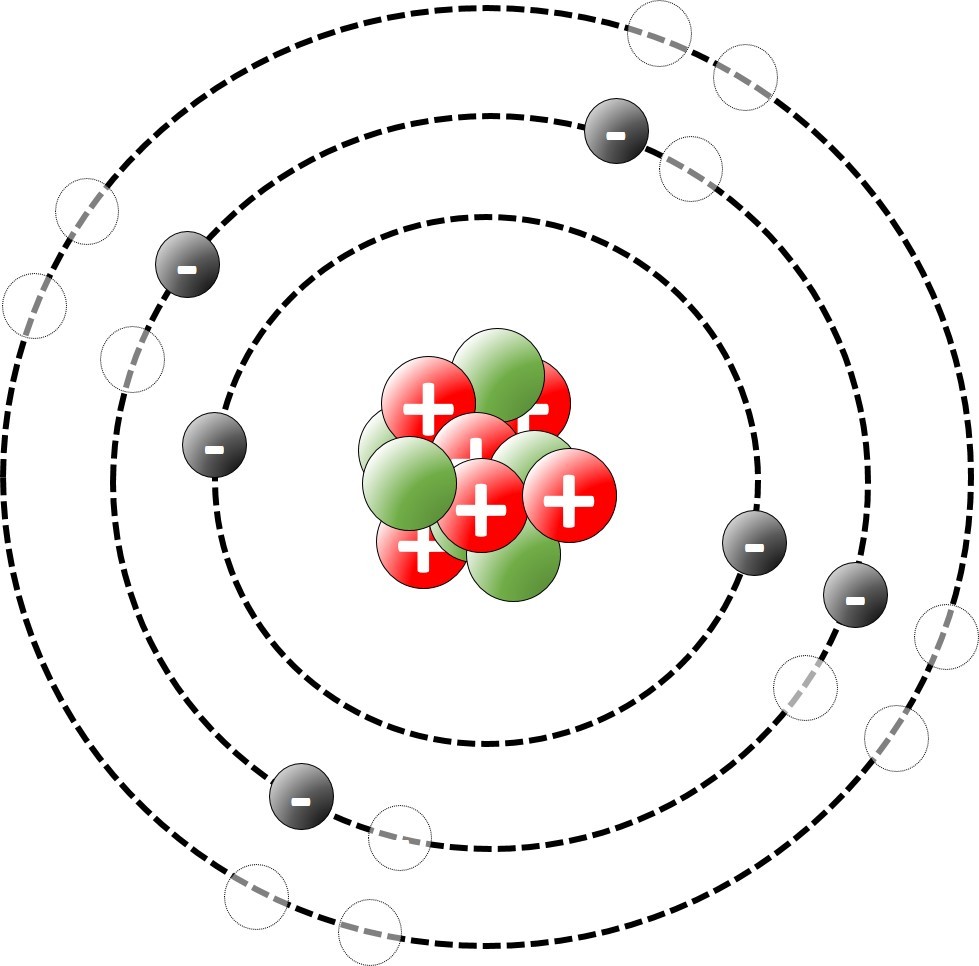
The modern model of the atom suggests that virtually all of the mass of an atom is concentrated in the middle ( the nucleus). The nucleus is surrounded by electrons.
Inner space
The small size of atoms give scientists a problem when trying to see them. Conventional microscopes cannot resolve particles at an atomic scale.
This short video gives an outline of how modern science is beginning to reveal the atomic world to us in a visual way.
The technique outlined involves a nanoparticle of the element platinum. This is a particle which is a few nanometres in size.
The nanometre (symbol: nm) is a unit used to measure length . It is equal to one billionth of a metre (1 m / 1,000,000,000).
1.14 Visualising atoms
Students should:
1.14 know what is meant by the terms atom and molecule
1.15 know the structure of an atom in terms of the positions, relative masses and relative charges of sub-atomic particles
The modern model of the atom was developed early in the 20th Century.
Sometimes it is convenient to think of atoms as spheres. On other occasions we think of a model where the atom is made up of a nucleus surrounded by shells of electrons.
An orbital model of the atom shows electrons in orbit around the nucleus.
1.14 Modelling molecules
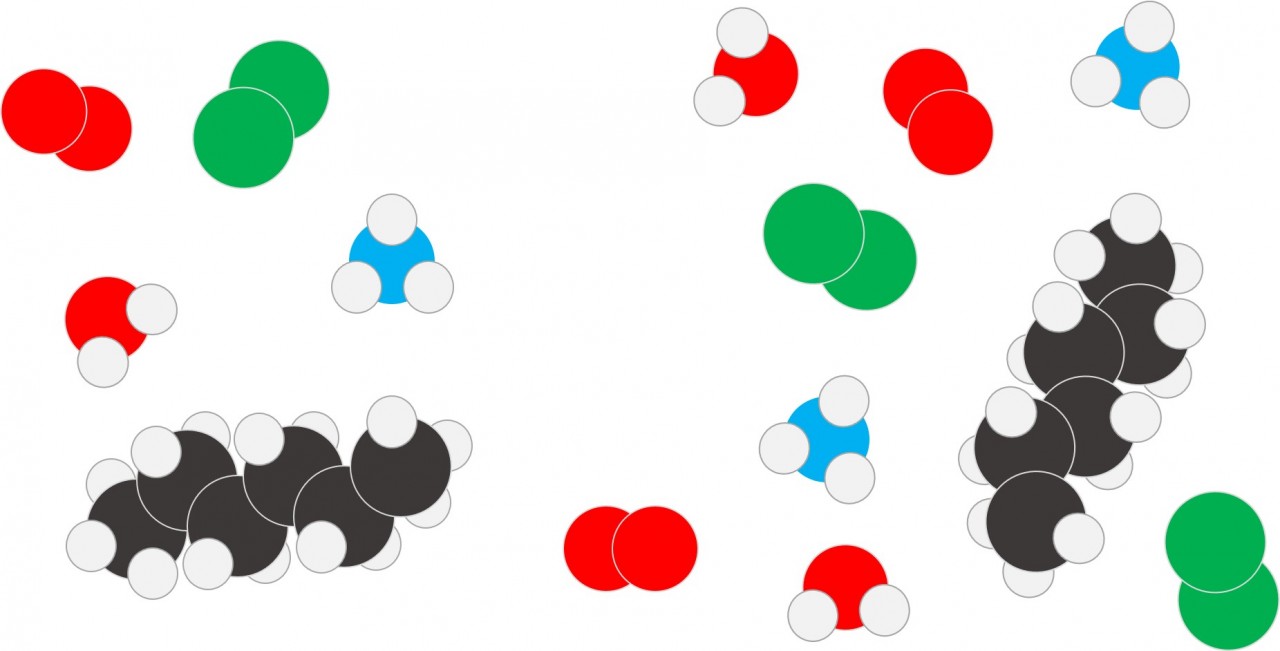
A molecule is a particle composed of more than one atom joined together by covalent bonds.
The atoms in a molecule might all be of the same element eg Hydrogen (H2) or Oxygen (O2) . When a molecule contains atoms of different elements it is a compound e.g. Water (H2O) or Methane (CH4).
The different colours are used to represent atoms of different elements:
| Red | Oxygen |
| White | Hydrogen |
| Blue | Nitrogen |
| Black | Carbon |
| Green | Chlorine |
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.