Crude Combustion and Consumption
If you're starting out on an A level chemistry course take a little time to revise the bits of chemistry below.
You will have performed calculations involving mass changes and you will have studied combustion reactions. In the video I've done a rough calculation to see how much we think we might have reduced our annual carbon dioxide production by switching from an old car running on diesel fuel to a new petrol/electric hybrid car.
HOW DO HYBRIDS HELP?
Simple really - they burn less fuel so they produce less Carbon Dioxide...
But is that all there is to it?
Watch the video, follow the equations and calculations. They should make sense to you - but if not, ask for some help.
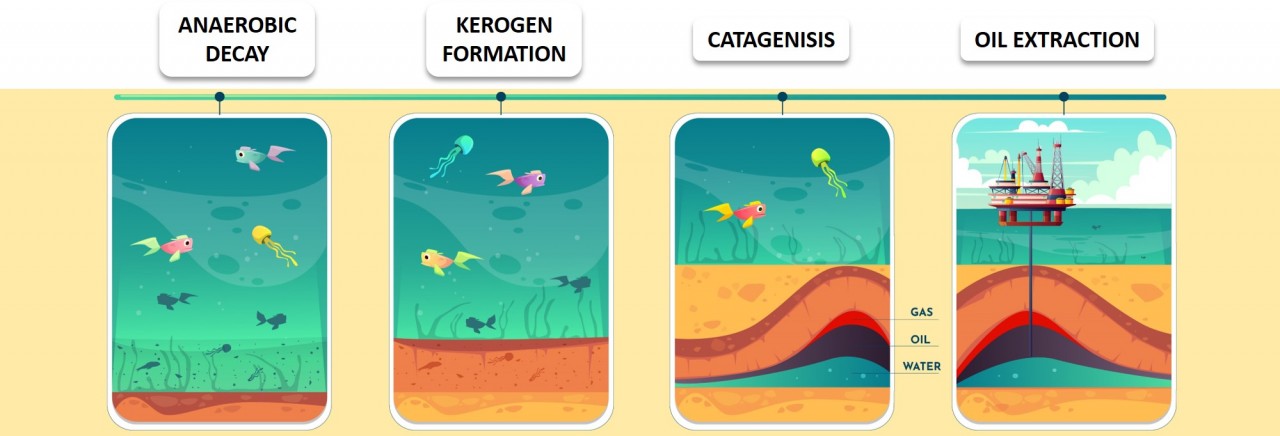
black gold
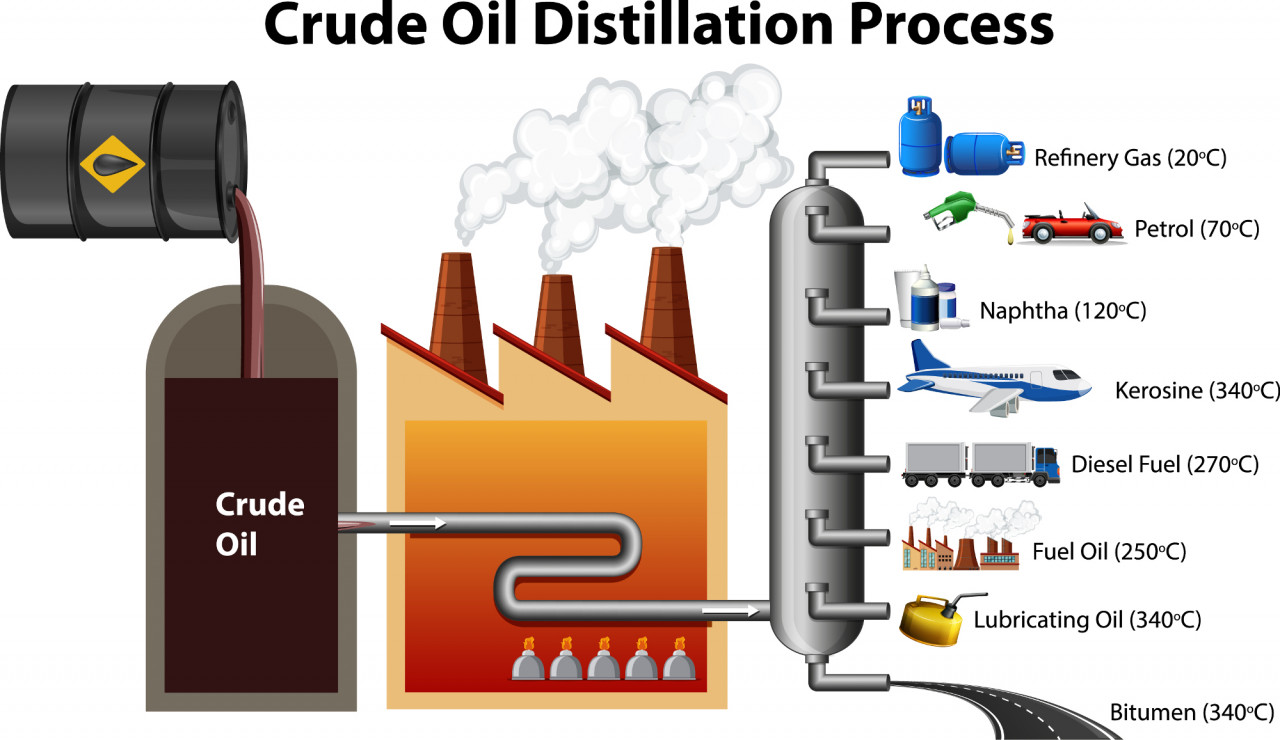
Ancient marine animals trapped in sedimentary rocks millions of years ago decomposed to form a mixture of hydrocarbons known as crude oil. Crude oil is found in underground reservoirs, trapped in rock formations. It is pumped up to the surface before being refined.
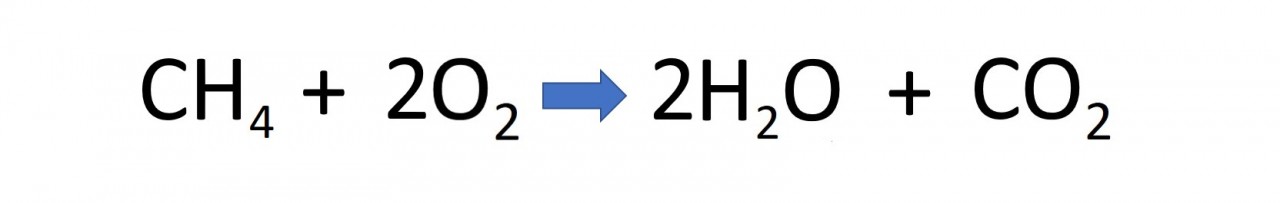
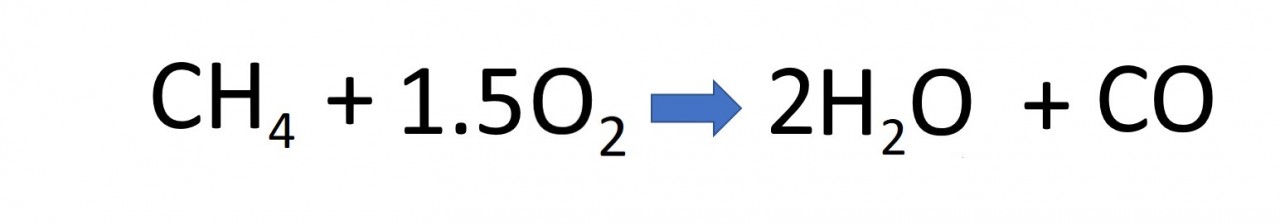
complete combustion
The hydrocarbons obtained from crude oil are combustible. They will burn in the oxygen of the air to produce the oxides of hydrogen ( water ) and carbon. The reaction releases energy and is therefore exothermic.