The Odessa port plant in the Ukraine produces ammonia and other nitrogenous fertilisers.
Scientists agree that too much carbon dioxide is still being released into the atmosphere by human activity. Yet in this recent article, the BBC report on how a shortage of carbon dioxide is causing concern for meat producers and consumers amongst others....
Carbon dioxide - some facts and questions
Carbon dioxide is a by product of ammonia production
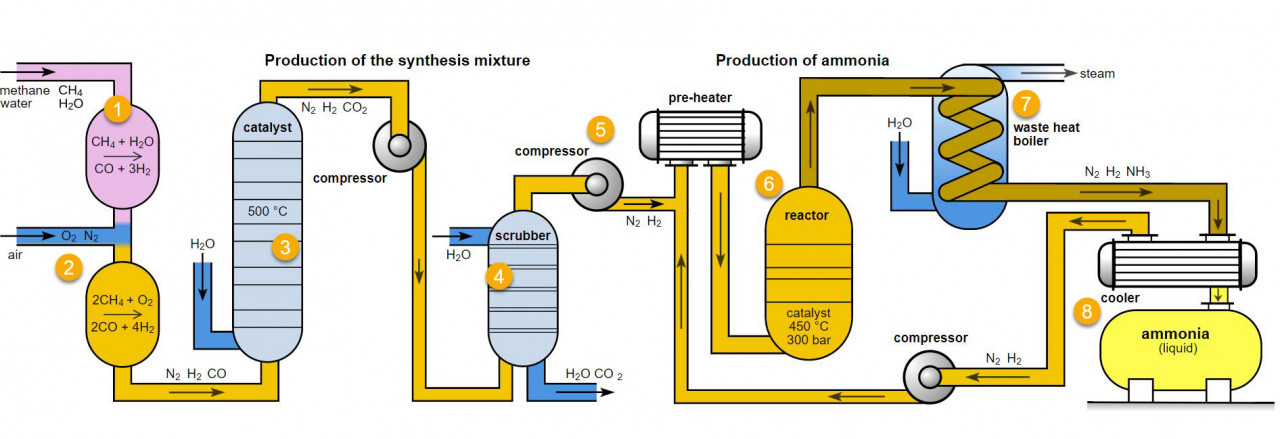
If you study the flow chart for ammonia production below you will note that in the scrubber, carbon dioxide is removed from the reaction mixture. The carbon dioxide produced is in quite a pure form so it can then be transported for use in the drinks and meat industries.
Carbon dioxide is used to stun animals before slaughter
- Why is carbon dioxide used this way?
- Is it the most humane option ?
- Are there any alternative ways that animals could be prepared for slaughter?
Carbon dioxide is used to make fizzy drinks
- Do we have to have bubbles in our drinks?
- Why is carbon dioxide used rather than another gas such as nitrogen?
You can find a lot of discussion about these questions at:
Why not extract carbon dioxide from the atmosphere ?
Carbon dioxide makes up only 0.04% of the atmosphere. It can be extracted from the atmosphere by cooling. But this this will require huge amounts of energy and so producing carbon dioxide in this way is not ( yet ) economically viable.
Water + Gas + Air = fertiliser ( GCSE chemistry)
If studying GCSE - you should work your way through this presentation. If studying A level - you might like to run through it by way of revision..
Why soaring gas prices have reduced ammonia production..
Natural gas ( Methane CH4) is one of the raw materials for the production of ammonia.
A recent massive rise in the price of wholesale natural gas and a decrease in the demand for (and therefore price of) ammonia has meant that ammonia manufacture has declined and in some places has been halted.
Carbon dioxide is a by product of ammonia production.
This has led to a decrease in supply of commercial carbon dioxide and caused the current shortage.
1. What are the raw materials for the production of ammonia?
2. Which elements are present in ammonia and in what ratio?
Nitrogen and hydrogen in a ratio of 1:3
3. Which of the elements are therefore removed ?
Carbon and oxygen in the form of CO2
Some important chemical principles
the ammonia production process
Various parts of this process are frequently used for examples which help to explain principles of equilibria, reaction rates, catalysts and
atom economy. All of these ideas can be found in A level chemistry . It is a good idea to become familiar with it. Parts 6, 7 and 8 are usually studied at GCSE.
Key concept : Atom economy
The atom economy of a reaction is a measure of the amount of starting materials that end up as useful products. It is important for sustainable development and for economic reasons to use reactions with high atom economy.
In this video David shows how the atom economy for stage 1 of the ammonia production process can be calculated.