1.40 - 1.43 Ionic Bonding - properties
1.34 understand that ionic compounds have high melting and boiling points because of strong electrostatic forces between oppositely charged ions
1.35 understand the relationship between ionic charge and the melting point and boiling point of an ionic compound
1.36 describe an ionic crystal as a giant three-dimensional lattice structure held together by the attraction between oppositely charged ions
1.37 draw a diagram to represent the positions of the ions in a crystal of sodium chloride
Video Exercise : Watch the video and then answer the questions below
-
Q1. At what point does the salt start conducting? Open or Close
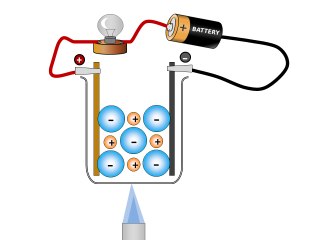
The salt begins to conduct an electric current once it has all become molten.
-
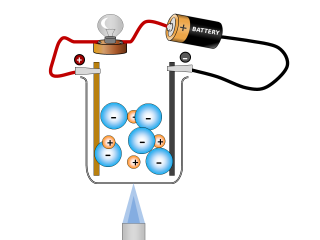
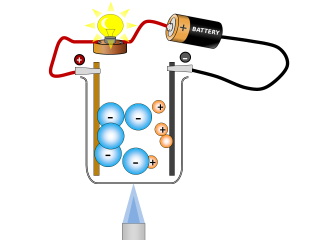
Q2. What changes can you see taking place in the sodium chloride whilst it is conducting? Open or Close
It looks like the salt is reacting and producing some gases
-
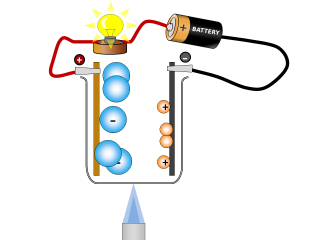
Q3. Why does the lamp go out again? Open or Close
The molten salt is allowed to cool and so it solidifies and stops being able to conduct
Why do ionic compounds conduct when molten?
FMCP !!
A rather more animated explanation from Richard Thornley
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.